Hampton Research蛋白结晶试剂盒






Products > Optimize Reagents > Optimize – Reducing Agent > TCEP hydrochloride
TCEP hydrochloride
Applications
- Stable reducing agent for crystallization trials
Features
- Highly soluble
- Odorless and non-volatile
- Effective at acidic and alkaline pH
Description
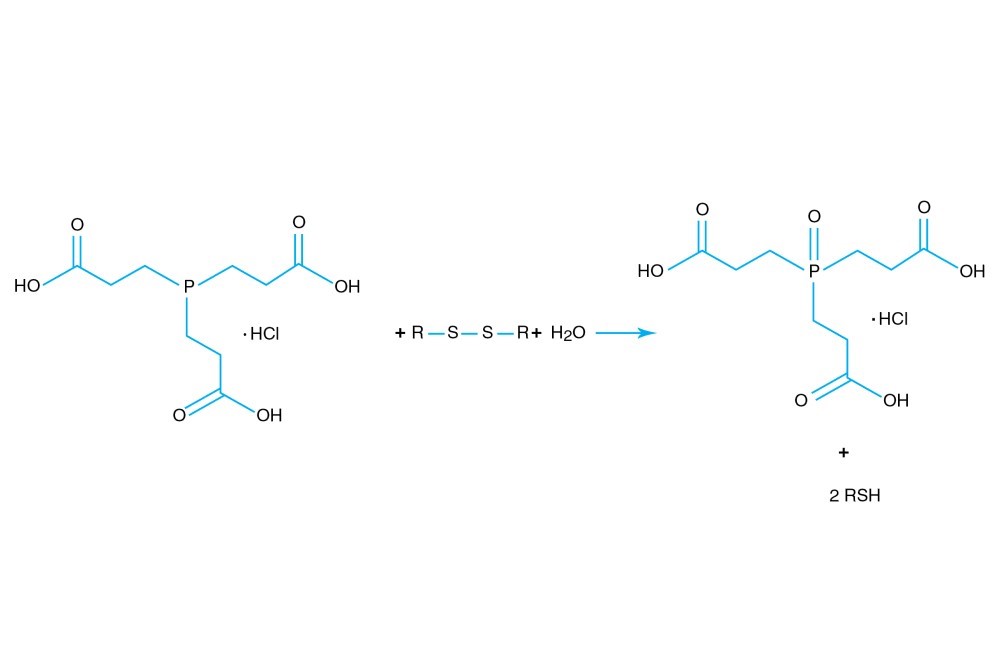
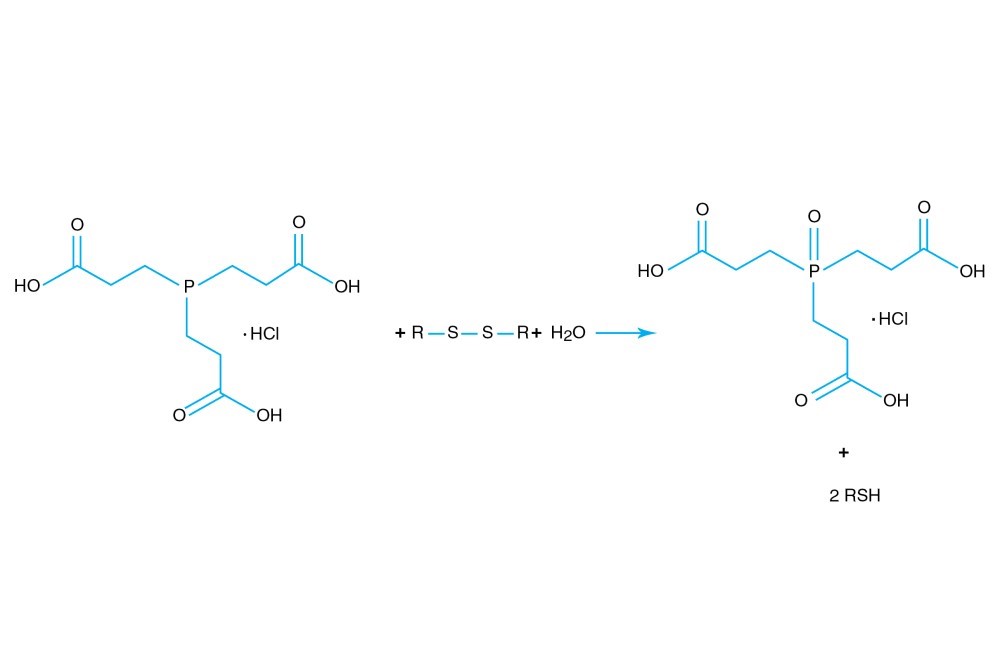
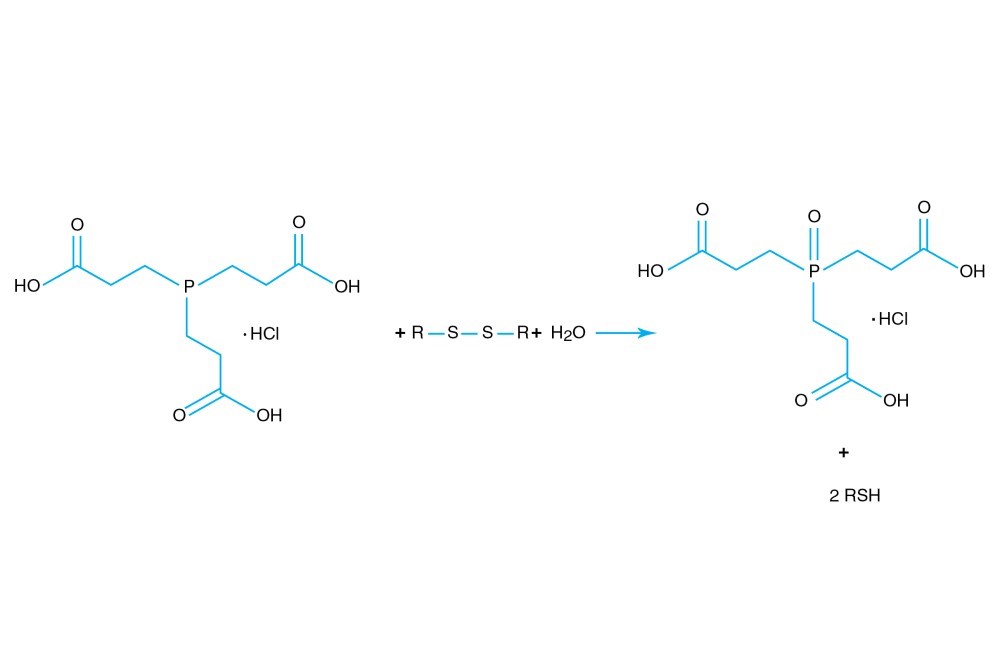
TCEP hydrochloride is Tri(2-carboxyethyl)phosphine hydro-chloride.
TCEP hydrochloride is an odorless, non-volatile reducing agent that is more stable and effective than DTT or 2-Mercaptoethanol. Unlike DTT, TCEP hydrochloride retains its reducing power at acid pHs (pH 5) and at pHs above 7.5 and is stable in air. TCEP hydrochloride is soluble in water to 310 grams per liter. For most applications, 5-50 mM TCEP provides sufficient molar excess to effectively reduce peptide or protein disulfide bonds within a few minutes at room temperature. TCEP hydrochloride is unreactive toward other functional groups found in proteins. Unlike DTT (dithiothreitol), TCEP hydrochloride does not contain a free thiol, and therefore does not require removal before reaction with thiol-reactive reagents. TCEP reduces disulfides but apparently not mercury-thiol bonds. TCEP hydrochloride is compatible with many heavy atoms and may be used during heavy atom derivatization. TCEP hydrochloride is more stable at a higher pH and at higher temperatures than is DTT and for a longer period of time in buffers without metal chelators such as EGTA. With TCEP, removal of the reducing agent is not necessary prior to most applications, (e.g. histidine-tagged protein purification, protein crystallization).
TCEP is typically very soluble in aqueous buffers at nearly any pH. Therefore, working concentrations and 10X stock solutions may be readily prepared in most aqueous buffers. TCEP is stable in aqueous, acidic, and basic solutions. When TCEP is dissolved directly in water, the resulting pH is approximately 2.5. TCEP is not very stable in phosphate buffers, especially at neutral pH. Therefore, if TCEP is to be used in PBS buffers, prepare the working solution immediately before use. TCEP may be used as a substitute loading buffer for SDS-PAGE; use a final concentration of 50 mM TCEP. Since TCEP is charged in solution, it is not compatible for use in isoelectric focusing.
A 10 mM TCEP hydrochloride concentration can reduce the pH of a 100 mM buffer by approximately 0.5 pH units; 1 mM TCEP hydrochloride concentration reducing the pH of a 100 mM buffer by 0.05 pH units.
Stability in Solution
TCEP is stable in aqueous acidic and basic solutions. When TCEP is dissolved directly in water the resulting pH is 2.5. Various buffers may be used for the reductions. Studies indicate that no change in concentration occurs after 24 hour incubation at room temperature in 100 mM HCL, 100 mM NaOH, or any of the following 50 mM buffers Tris HCl (pH 7.5, 8.5, 9.5), HEPES(pH 6.8, 8.2), Borate(pH 8.2, 10.2), and CAPS (pH 9.7, 11.1). Even after weeks in these buffers, less than 20% of the TCEP was oxidized.
TCEP is not particularly stable in phosphate buffers, especially at neutral pH. Experiments indicate that TCEP completely oxidizes within 72 hours in 0.35M phosphate-buffered saline (PBS), pH 7.0. Approximately 50% oxidation occurs in the same amount of time in 0.15M PBS, pH 8.0. Only minimal oxidation occurs in PBS at pH > 10.5 or < 6.0. Therefore, if TCEP is to be used in PBS buffers, prepare the working solution immediately before use.
Solid TCEP hydrochloride is supplied packaged in argon to minimize oxidation.
Molecular formula C9H15O6P • HCl
Molecular weight 286.65
CAS [51805-45-9]
Beilstein Registry Number 3724376
Purity ≥99.0%
Stable at room temperature for up to 2 years after receipt.
Click to Zoom In
CAT NO
HR2-651
NAME
DESCRIPTION
1 gram
PRICE
$29.00
cart quote
CAT NO
HR2-801
NAME
DESCRIPTION
10 grams
PRICE
$165.00
cart quote
Support Material(s)
 HR2-651 HR2-801 TCEP HCl User Guide
HR2-651 HR2-801 TCEP HCl User Guide HR2-651 TCEP hydrochloride SDS
HR2-651 TCEP hydrochloride SDS HR2-801 TCEP hydrochloride SDS
HR2-801 TCEP hydrochloride SDS Certificate Of Analysis
References
1. Tris(2-carboxyethyl)phosphine stabilization of RNA: comparison with dithiothreitol for use with nucleic acid and thiophosphoryl chemistry. Rhee SS, Burke DH. Anal Biochem 325, 137-43 (2004) PN51541.
2. A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Getz EB, Xiao M, Chakrabarty T, Cooke R, Selvin PR. Anal Biochem 273, 73-80 (1999) PN34481.
3. Burns, J.A., et al. (1991). Selective reduction of disulfides by tris-(2-carboxyethyl)-phosphine. J. Org. Chem. 56, 2648-2650.
4. Oda , Y., et al. (2001). Nature Biotech19, 379-382.


Hampton Research, first in crystallization since 1991, developing and delivering crystallization and optimization screens, reagents, plates, and other tools for the crystallization of biological macromolecules, including proteins (antibody), peptides (insulin), and nucleic acids (DNA).
- Products
- Gallery
- My Account
|
|
|
- Contact Us
- Quick Order
- Support
|
- Privacy Policy
- Terms and Conditions
|
- Products
- Gallery
- My Account
- Support
- Contact Us
- Quick Order
- Privacy Policy
- Terms and Conditions
|
|
|
|
|
|
|
© 2021 HAMPTON RESEARCH CORP.
| Website by Skyhound Internet