- 产品特性
- 相关资料
- Q&A
- 参考文献
蛋白聚集小体检测试剂盒![]()
PROTEOSTAT® Aggresome detection kit
◆原理
聚集小体(Aggresome)是由一群不正常堆叠在一起的蛋白质所形成的包涵体(Inclusion Bodies)。聚集小体的出现往往也表明细胞正处于某种应激状态,比如高温、病毒感染、活性氧自由基攻击等。聚集体通常是响应细胞应激而形成,并通过分离错误折叠的蛋白质提供细胞保护机制,最终导致它们被自噬清除。此外,蛋白质聚集体的形成是多种人类疾病的标志,例如阿尔茨海默氏症、帕金森氏症、肌萎缩侧索硬化症或酒精性肝病。
PROTEOSTAT® Aggresome detection kit中包含的 PROTEOSTAT® 是一种分子转子染料,在溶液中沿着单个中心键的自由分子内旋转可防止荧光产生。当它特异地插入到错误折叠和聚集的蛋白中的交叉β-sheet四级结构中,旋转受到抑制并导致发出强烈的荧光。
PROTEOSTAT® 聚集小体检测试剂盒提供了一种快速、特异、定量的方法来标记细胞中的聚集小体,且无需进行人工蛋白定点突变。PROTEOSTAT® 染料已在广泛的条件下对多种小分子调节剂进行验证,适用于具有治疗价值的化学物的筛选。此外,本试剂盒还适用于多重免疫荧光,以在自噬和聚集体形成的背景下研究您的目标分子。
◆试剂盒组成
|
组分 |
包装 |
|
PROTEOSTAT® Aggresome Detection Reagent |
10 μL |
|
Hoechst 33342 Nuclear Stain |
50 μL |
|
Proteasome Inhibitor (MG-132) |
120 nM |
|
10×Assay Buffer |
25 mL |
◆特点
● 提供基于细胞的灵敏的药物反应性测定,以在真实的细胞环境中识别与神经退行性疾病相关的抑制剂
● 可靠且简单的检测,不需要非生理性蛋白质突变或基因工程细胞系
● 作用条件广泛,适用于筛选具有潜在治疗价值的小分子化合物
● 已进行优化以兼容免疫染色
● 可通过流式细胞术轻松量化聚集体和相关包涵体
● 可用于神经退行性疾病、肝病、毒理学研究等的研究
◆案例・应用
■ 应用实例 1:流式细胞术检测聚集小体
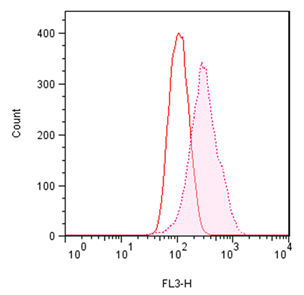
流式细胞术的分析:Jurkat细胞在37°C下用 0.2% DMSO对照或用5 µM MG-132诱导过夜。处理后,将细胞固定并与PROTEOSTAT® 染料一起孵育,无需洗涤即通过流式细胞术在FL3通道中使用488 nm激光进行分析。在 MG-132处理的细胞中,红色荧光信号增加约3倍。所描述的测定允许评估蛋白质聚集的影响。
■ 应用实例 2:PROTEOSTAT® 染料与荧光染料的应用
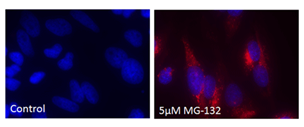
荧光显微镜:含有蛋白聚集体的HeLa细胞,用 5 µM MG-132蛋白酶体抑制剂(右)处理12小时,通过 PROTEOSTAT®(红色)检测并用Hoechst 33342复染。左侧的为未经处理的对照。
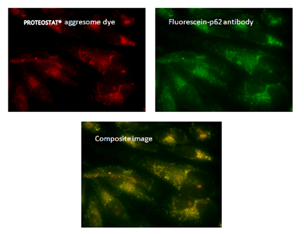
荧光显微镜:含有蛋白聚集体的HeLa细胞,用 5μM MG-132蛋白酶体抑制剂处理12小时,通过PROTEOSTAT® 聚集体染料(左上)检测,显示与荧光素-p62抗体(右上)和复合图像(中下)的共定位
■ 应用实例 3:不同搅拌速率对蛋白聚集的影响
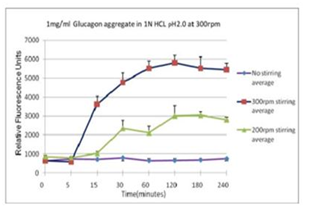
使用本产品检测后,可知搅拌速度越快,蛋白聚集程度越大。
■ 应用实例 4:蛋氨酸氧化可抑制蛋白聚集
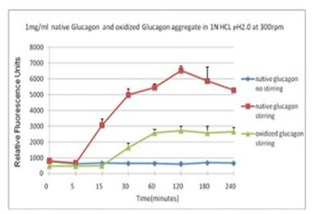
蓝色曲线为对照,没有搅拌;在搅拌速度在300 rpm下, 绿色曲线与红色曲线分别表示蛋氨酸被氧化后的蛋白聚集情况。
使用本产品检测后,可知蛋氨酸被氧化后可抑制蛋白聚集。
■ 应用实例 5 :内质网应激相关细胞自噬产生蛋白聚集小体
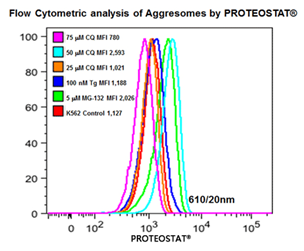
分别25,50,75 mmCQ,100 nM的thapsigargin(TG)或5mM的MG-132处理K562细胞24小时。然后在温室下用PROTEOSTAT® (1:10,000)固定并透化细胞30min。接着在BD流式细胞仪LSR II的蓝色610/20 nm的通道分析细胞(30,000)。
图片来源:Courtesy of the Flow Cytometry Core Facility, Blizard Institute, Queen Mary University of London, London, UK.
■ 应用实例 6
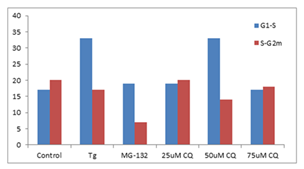
K562 细胞分别使用Thapsigargin (Tg, 0.1 µM)、25、50 和 75 µM 氯喹 (CQ) 用试剂处理以诱导内质网应激和不完全自噬 24 h。使用蛋白酶体抑制剂 MG-132 (5 µM) 作为阳性对照处理24 h。将沉淀的细胞固定并透化。然后,根据制造商的说明,使用 300 µL PROTEOSTAT® 和 1 µg/mL DAPI(用于细胞周期测定)在室温下标记细胞30min。确定每个处理的S期的 Aggresome 倾向因子 (APF) 高于G1和高于S期的G2m的相对增加,并与对照值进行比较。与对照组相比,ER 应激诱导剂、Tg 和 50 µM CQ在S期比G1更能引起蛋白聚集体上调,但蛋白酶体抑制剂 MG-132 和 50 µM CQ 在 G2m 期比S期更显著地下调聚集体。
图片来源:Courtesy of the Flow Cytometry Core Facility, Blizard Institute, Queen Mary University of London, London, UK.
PROTEOSTAT® Aggresome Detection Kit
PROTEOSTAT® 蛋白聚集检测试剂盒 Q&A
1.Q: PROTEOSTAT® 热稳定检测试剂盒(ENZ-51027),蛋白聚集检测试剂盒(ENZ-51023)
1.Q: 和PROTEOSTAT® 蛋白聚集检测试剂盒(ENZ-51035)的区别?
1.A: 1)PROTEOSTAT®染料属于分子轮子染料(molecular rotor dye)。在溶液状态不发荧光(由于在中心的碳-碳单键周围的自由转动,
分离了探针的不同芳香部分)。由于分子轮子染料接触到蛋白聚集体后,会封闭进入原纤维,形成β折叠,具有高强度荧光。类似于
染核酸碱基的EB 一样。
1.A: 2)PROTEOSTAT® 热稳定检测试剂盒(ENZ-51027)用于直接监测热诱导蛋白变性引起的蛋白聚集,不检测蛋白非折叠而暴露的疏
水部分。该试剂盒用于检测蛋白缓冲液,其他添加成分对蛋白稳定性的影响,在特定条件下多少温度时蛋白会聚集。
1. A 3)蛋白聚集检测试剂盒(ENZ-51023)用于检测液体状蛋白或者多肽形成聚集的情况。可用于确认蛋白保存的最优形态,筛选促进或
者抑制蛋白聚集的试剂,检测分子伴侣的活性。与标准品或者已知浓度样品一起使用,可定量检测蛋白聚集。
1. A 4)PROTEOSTAT® 蛋白聚集检测试剂盒(ENZ-51035)检测变性蛋白,包括活细胞内聚集体或者聚集体样包涵体。PROTEOSTAT®
蛋白聚集检测染料在聚集小体形成时,接触到聚集蛋白时会变的更明亮,可用流式或者荧光显微镜检测。
2.Q: 使用ENZ-51035 时,是否可以终止并在在40℃过夜保存,第二天继续实验?
A: 建议可以在加染料前终止。荧光强度在液体中会成倍降低。而且染料与蛋白长时间孵育后,会影响蛋白聚集。
3.Q: 阳性对照染色不好。
A: 染料没有避光保存。染色时候没有避光,需要染色后立即检测。聚集蛋白不是液体。延长离心会使聚集蛋白沉淀。不要离心聚集蛋
白(样品或者对照)。
4.Q: 蛋白信号饱和。
A: 蛋白样品的浓度很高。用1×Assay Buffer 稀释样品。
5.Q: 阴性对照观察到高荧光强度。
A: 样品含有干扰物质。该试剂可以与常规使用的缓冲液(PBS,Tris,HEPES)和赋形剂(海藻糖和蔗糖)使用,不过不要使用高浓度的吐温
20(比如:0.2%)
6.Q: 蛋白与PROTEOSTAT® 检测试剂结合是否是可逆的?
A: PROTEOSTAT® 检测试剂与聚集蛋白的相互作用是非供价结合。所以,理论上是可逆的。但是我们没有尝试将染料从样品中去除。
7.Q: 在药物应激检测中,细胞是否可以经胰酶消化后去检测细胞中的蛋白聚集?
A: 胰酶可以与PROTEOSTAT® 聚集检测试剂盒配套使用。细胞消化后,表面活性剂加入给细胞打孔。更大的聚集体仍然是不溶的,可
以通过离心沉淀。小的聚集体通过超滤分离。表面活性剂,膜和可溶蛋白可洗掉,PROTEOSTAT® 检测试剂可用于残留蛋白。
8.Q: 在研究细胞蛋白聚集时,如何设置阳性对照?
A: MG 132 处理的细胞可作为阳性对照。
9.Q: 细胞裂解液是否可用PROTEOSTAT® 蛋白聚集检测试剂检测?
A: 检测细胞裂解液是有挑战的,表面活性剂会引起高背景。可以过滤去除表面活性剂。更大的聚集体仍然是不可溶的,可以通过
离心沉淀。小的聚集体通过超滤分离。表面活性剂,膜和可溶蛋白可洗掉,PROTEOSTAT® 检测试剂可用于残留蛋白。
10.Q: PROTEOSTAT® 染料是否可以检测~300kDa 的蛋白二聚体?
A: PROTEOSTAT® 可以检测从单体到二聚体转变的蛋白,结合分子筛层析法。但是信号会变的更强烈,因为聚集体更大。
11.Q: 如果样品稀释到低浓度(比如0.5mg/mL)是否会改变聚集蛋白的百分比含量?
A: 聚集蛋白的百分比(聚集蛋白占总蛋白的比例)将保持不变。聚集蛋白的浓度会降低,信号也会减弱。
A: 如果用未聚集蛋白稀释样品,聚体蛋白的百分比会变化。
12.Q: PROTEOSTAT® 试剂盒是否可用于研究原核细胞样品的蛋白聚集?
A: 如果膜部分去除的话,是可以检测原核细胞的。如果膜没有分离,染料会聚集在膜上,引起高背景值。
13.Q: PROTEOSTAT® 是否可检测革兰氏阴性菌的蛋白聚集?
A: 革兰氏阴性菌外层膜的脂多糖会影响。操作手册中提到的打孔缓冲液将移除大多数细菌的内层膜,破坏革兰氏阴性菌的外层膜。所以
可以观察革兰氏阴性菌的聚集和包涵体。
14.Q: 当其他蛋白共染时,如何优化减少FITC 发射过滤装置的信号?
A: 染料的激发和发射光谱见操作手册P8。光谱显示会与FITC 有些重叠。FITC 可以与该染料一起使用,用488nm激发波长和515nm 发
射波长。如果FITC 有荧光的话就会起作用。否则,PROTEOSTAT® 染料会有溢出。建立设立一个不带有FITC 标记的抗体作为对照。
另外,可用Cy5 或者Coumarin 标记的抗体解决这个问题。
|
1. |
A fast and specific method to screen for intracellular amyloid inhibitors using bacterial model systems: S. Navarro, et al.; Eur. J. Med. Chem. (2015), Application(s): Confocal microscopy, Abstract; |
|
2. |
Amyloidogenic lysozymes accumulate in the endoplasmic reticulum accompanied by the augmentation of ER stress signals: Y. Kamada, et al.; Biochim. Biophys. Acta 1850, 1107 (2015), Application(s): Microscopy, Abstract; Conophylline protects cells in cellular models of neurodegenerative diseases by inducing mammalian target of rapamycin (mTOR)-independent autophagy: Y. Sasazawa, et al.; J. Biol. Chem. 290, 6168 (2015), Abstract; |
|
3. |
Decreased proteasomal function accelerates cigarette smoke-induced pulmonary emphysema in mice: Y. Yamada, et al.; Lab. Invest. 95, 625 (2015), Application(s):Aggresome detection by fluorescence microscopy in fibroblasts, Abstract; |
|
4. |
Defective autophagy is a key feature of cerebral cavernous malformations: S. Marchi, et al.; EMBO Mol. Med. 7, 1403 (2015), Application(s): Aggresome detection in aggregated proteins and aggresome‐like inclusion bodies in fixed and permeabilized samples,Abstract; Full Text |
|
5. |
Fibril growth and seeding capacity play key roles in α-synuclein-mediated apoptotic cell death: A.L. Mahul-Mellier, et al.; Cell Death Differ. 22, 2107 (2015), Abstract; |
|
6. |
In vitro administration of gold nanoparticles functionalized with MUC-1 protein fragment generates anticancer vaccine response via macrophage activation and polarization mechanism: T. Mocan, et al.; J. Cancer 6, 583 (2015), Application(s): Aggresome detection by fluorescence microscopy in peritoneal macrophages, Abstract; Full Text |
|
7. |
Intensified autophagy compromises the efficacy of radiotherapy against prostate cancer: M.I. Koukourakis, et al.; Biochem. Biophys. Res. Commun. 461, 268 (2015),Application(s): Fluorescence microscopy , Abstract; Mevalonate pathway regulates cell size homeostasis and proteostasis through autophagy: T.P. Miettinen, et al.; Cell Rep. 13, 2610 (2015), Application(s): Flow cytometry analysis of protein aggregation using Jurkat, U2OS, Kc167 and HUVEC cells, Abstract; |
|
8. |
MiR-29b replacement inhibits proteasomes and disrupts aggresome+autophagosome formation to enhance the antimyeloma benefit of bortezomib: S. Jagannathan, et al.; Leukemia 29, 727 (2015), Application(s): Detection of protein aggregates by fluorescence microscopy in multiple myeloma cell lines, Abstract; Full Text |
|
9. |
Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma: M.A. Abdel Malek, et al.; Oncotarget 6, 3098 (2015), Application(s): Confocal Microscopy, Abstract; Full Text |
|
10. |
Monitoring of dipeptidyl peptidase-IV (DPP-IV) activity in patients with mucopolysaccharidoses types I and II on enzyme replacement therapy – Results of a pilot study: K. Hetmanczyk, et al.; Clin. Biochem. (2015), Application(s): Plasma DPP-IV enzyme assay, Abstract; |
|
11. |
Pressure overload-induced cardiac dysfunction in aged male adiponectin knockout mice is associated with autophagy deficiency: J.W. Jahng, et al.; Endocrinology 156, 1667 (2015),Abstract; Protein kinase C-dependent growth-associated protein 43 phosphorylation regulates gephyrin aggregation at developing GABAergic synapses: C.Y. Wang, et al.; Mol. Cell. Biol.35, 1712 (2015), Abstract; Schwann cells contribute to neurodegeneration in transthyretin amyloidosis: T. Murakami, et al.; J. Neurochem. 134, 66 (2015), Abstract; |
|
12. |
Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress: H.W. Chiu, et al.; Nanoscale 7, 736 (2014), Abstract; Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen: S.M. Usmani, et al.; Nat. Commun. 5, 3508 (2014), Application: Amyloid detection in semen,Abstract; |
|
13. |
Distinct patterns of HSP30 and HSP70 degradation in Xenopus laevis A6 cells recovering from thermal stress: S. Khan, et al.; Comp. Biochem. Physiol. A Mol. Integr. Physiol. 168, 1 (2014), Application(s): Detection of aggresomes in Xenopus laevis cells using fluorescence microscopy, Abstract; Dynein function and protein clearance changes in tumor cells induced by a kunitz-type molecule, amblyomin-x: M.T. Pacheco, et al.; PLoS One 9, e111907 (2014), Application(s):Detection of aggresomes by flow cytometry, Abstract; Full Text |
|
14. |
Higher vulnerability and stress sensitivity of neuronal precursor cells carrying an alpha-synuclein gene triplication: A. Flierl, et al.; PLoS One 9, e112413 (2014), Application(s):Detection of protein aggregates by fluorescence microscopy and flow cytometry in neuronal precursor cells, Abstract; Full Text |
|
15. |
Human stefin B role in cell's response to misfolded proteins and autophagy: M. Polajnar, et al.; PLoS One 9, e102500 (2014), Application(s): Detection of protein aggregates in primary astrocytes, Abstract; Full Text |
|
16. |
Novel estradiol analogue induces apoptosis and autophagy in esophageal carcinoma cells: E. Wolmarans, et al.; Cell Mol. Biol. Lett. 19, 98 (2014), Abstract; |
|
17. |
Preconditioning stimulus of proteasome inhibitor enhances aggresome formation and autophagy in differentiated SH-SY5Y cells: Y. Bang, et al.; Neurosci. Lett. 566, 263 (2014),Abstract; |
|
18. |
Protein deubiquitination during oocyte maturation influences sperm function during fertilisation, antipolyspermy defense and embryo development: Y.J. Yi, et al.; Reprod. Fertil. Dev. (2014), Application(s): Detection of protein aggregates in oocytes, Abstract; |
|
19. |
Protein expression pattern of PAWP in bull spermatozoa is associated with sperm quality and fertility following artificial insemination: C.E. Kennedy, et al.; Mol. Reprod. Dev. 81, 436 (2014), Abstract; Serine/threonine kinase 16 and MAL2 regulate constitutive secretion of soluble cargo in hepatic cells: J.G. In, et al.; Biochem. J. 463, 201 (2014), Abstract; |
|
20. |
SGTA regulates the cytosolic quality control of hydrophobic substrates: L. Wunderley, et al.; J. Cell. Sci. 127, 4728 (2014), Application(s): Dual staining with ProteoStat® dye, Abstract;Full Text |
|
21. |
T he small heat shock protein B8 (HSPB8) confers resistance to bortezomib by promoting autophagic removal of misfolded proteins in multiple myeloma cells: M. Hamouda, et al.; Oncotarget 5, 6252 (2014), Application(s): Analysis of velcade resistant multiple myeloma human cells by WB, Assay, Abstract; Full Text |
|
22. |
Aldosterone and angiotensin II induce protein aggregation in renal proximal tubules: M.U. Cheema, et al.; Physiol. Rep. 1, e00064 (2013), Application(s): Labeling of kidney homogenates, labeled particles sorted by flow cytometry and identification by LC-MS/MS ,Abstract; Full Text |
|
23. |
Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death: P. Magnaghi, et al.; Nat. Chem. Biol. 9, 548 (2013), Application(s): Detection of aggresomes in human colon carcinoma HCT116 cells using fluorescence microscopy, Abstract; |
|
24. |
Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner: Y. Nakajima, et al.; Int. J. Mol. Sci. 14, 7771 (2013),Application(s): Detection of aggresomes using fluorescence microscopy, Abstract; Full Text |
|
25. |
In vitro changes in mitochondrial potential, aggresome formation and caspase activity by a novel 17-β-estradiol analogue in breast adenocarcinoma cells: D.S. Nkandeu, et al.; Cell. Biochem. Funct. 31, 566 (2013), Abstract; Increased generation of cyclopentenone prostaglandins after brain ischemia and their role in aggregation of ubiquitinated proteins in neurons: H. Liu, et al.; Neurotox. Res. 24, 191 (2013), Abstract; |
|
26. |
Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells: S. Moriya, et al.; Int. J. Oncol.42, 1541 (2013), Application(s): Detection of aggresomes using flow cytometry, Abstract;Full Text |
|
27. |
Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2: Y. Maejima, et al.; Nat. Med. 19, 1478 (2013), Application(s): Detection of aggresomes in mouse heart sections using fluorescence microscopy, Abstract; Full Text |
|
28. |
N-terminally truncated forms of human cathepsin F accumulate in aggresome-like inclusions: B. Jeric, et al.; Biochim. Biophys. Acta 1833, 2254 (2013), Application(s):Detection of aggresomes using fluorescence microscopy, Abstract; |
|
29. |
The ubiquitin proteasome system regulates the stability and activity of the glucose sensor glucokinase in pancreatic beta cells: A. Hofmeister-Brix, et al.; Biochem. J. 456, 173 (2013),Abstract; Full Text |
|
30. |
VCP Phosphorylation-Dependent Interaction Partners Prevent Apoptosis in Helicobacter pylori-Infected Gastric Epithelial Cells: C.C. Yu, et al.; PLoS One 8, e55724 (2013),Application(s): Aggresome detection in AGS human gastric epithelial cells, Abstract; Full Text |
|
31. |
Zerumbone, an electrophilic sesquiterpene, induces cellular proteo-stress leading to activation of ubiquitin-proteasome system and autophagy: K. Ohnishi, et al.; BBRC 430, 616 (2013), Application(s): Aggresome detection in mouse hepatocytes, Abstract; |
|
32. |
Autophagy in idiopathic pulmonary fibrosis: A.S. Patel, et al.; PLoS One 7, e41394 (2012),Application(s): Detection of aggresomes in lung tissue sections using fluorescence microscopy, Abstract; Full Text |
|
33. |
Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities: U. Tomaru, et al.; Am. J. Pathol. 180, 963 (2012),Abstract; |
|
34. |
Mutations in the area composita protein αT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy: J. van Hengel, et al.; Eur. Heart J. 34, 201 (2012),Abstract; |
|
35. |
Quantitative analysis of α-synuclein solubility in living cells using split GFP complementation: A. Kothawala, et al.; PLoS One 7, e43505 (2012), Application(s):Aggresome detection in HeLa cells, Abstract; Full Text |
|
36. |
Multiple aggregates and aggresomes of C-terminal truncated human αA-crystallins in mammalian cells and protection by αB-crystallin: I. Raju, et al.; PLoS One 6, e19876 (2011), Application(s): Aggresome detection in HeLa cells, Abstract; Full Text |
|
37. |
Novel Cell- and Tissue-Based Assays for Detecting Misfolded and Aggregated Protein Accumulation Within Aggresomes and Inclusion Bodies: D. Shen, et al.; Cell Biochem. Biophys. 60, 173 (2011), Abstract; Full Text |
|
38. |
Inhibitors of protein aggregation and toxicity: H. Amijee, et al.; Biochem. Soc. Trans. 37, 692 (2009), Abstract; Full Text |
|
39. |
Autophagy-mediated clearance of aggresomes is not a universal phenomenon: E. Wong, et al.; Hum. Mol. Genet. 17, 2570 (2008), Abstract; Full Text |
|
40. |
Chemical and biological approaches synergize to ameliorate protein-folding diseases: T.W. Mu, et al. ; Cell 134, 769 (2008), Abstract; Full Text |
|
41. |
Inhibitors of the proteasome suppress homologous DNA recombination in mammalian cells: Y. Murakawa, et al.; Cancer Res. 67, 8536 (2007), Abstract; |
|
42. |
p62SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death: G. Bjørkøy, et al.; J.Cell Biol. 171, 603 (2005), Full Text |
|
43. |
Therapeutic effects of cystamine in a murine model of Huntington's disease: A. Dedeoglu, et al.; J. Neurosci. 22, 8942 (2002), Full Text |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 |
| ENZ-51035-K100 | PROTEOSTAT® Aggresome detection kit PROTEOSTAT®蛋白聚集小体检测试剂盒 |
100 tests | – |
| ENZ-51035-0025 | PROTEOSTAT® Aggresome detection kit PROTEOSTAT® 蛋白聚集小体检测试剂盒 |
25 tests | – |
